Liver metastases are the key determinant of overall survival in metastatic uveal melanoma.1

Recent data from the FOCUS study shows that more than 1/3 of patients responded to treatment with Chemosat (ORR reported in FOCUS was 36.3%, n = 33)2
Liver directed therapy is the recommended treatment approach for patients with hepatic dominant uveal melanoma, where resection is not possible.3,4
Liver metastases are the key determinant of overall survival in metastatic uveal melanoma.1
Recent data from the FOCUS study shows that more than 1/3 of patients responded to treatment with Chemosat (ORR reported in FOCUS was 36.3%, n = 33)2
Liver directed therapy is the recommended treatment approach for patients with hepatic dominant uveal melanoma, where resection is not possible.3,4
CHEMOSAT is a liver directed therapy with growing evidence to support its role as a standard of care in metastatic uveal melanoma.5-7
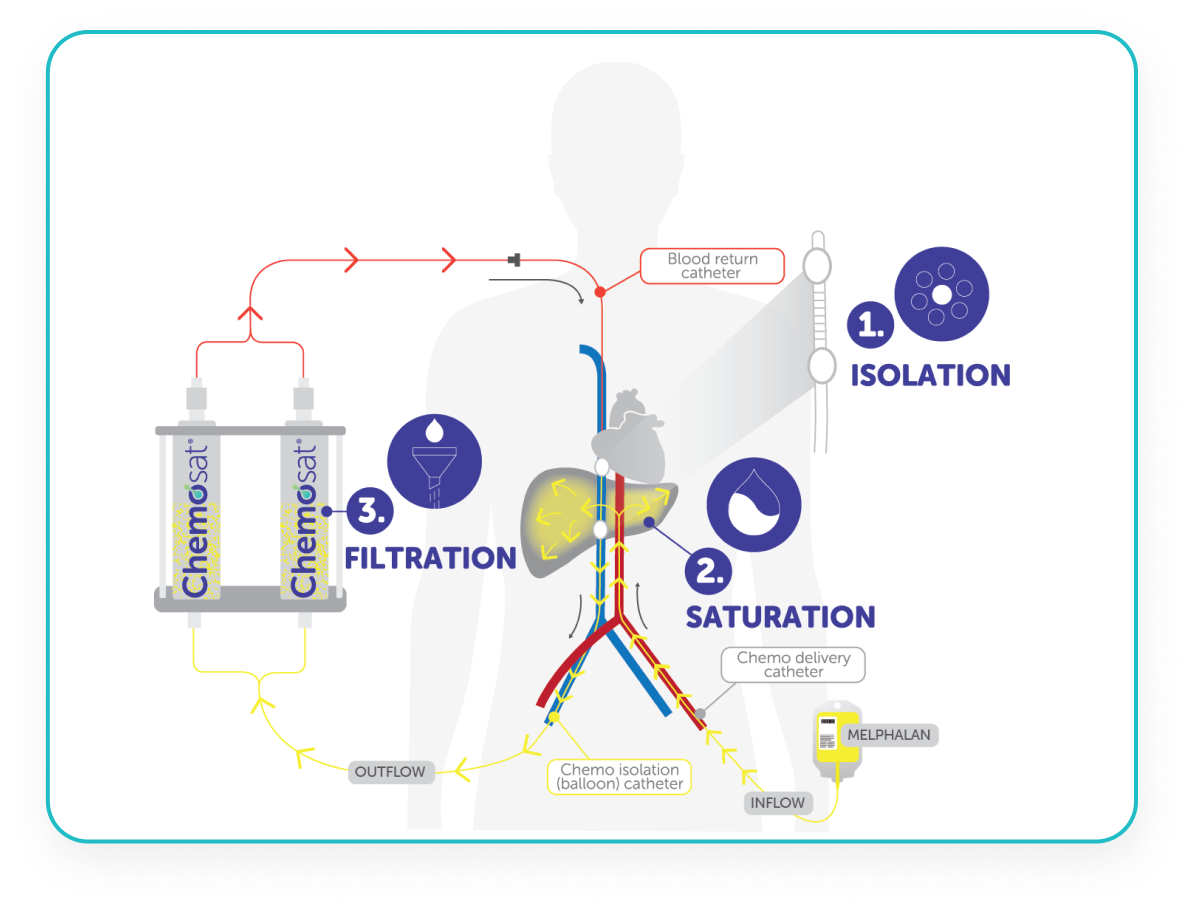
CHEMOSAT harnesses the tumour vascular supply by delivery of melphalan into the hepatic artery, which represents almost exclusively the arterial supply to liver tumours.5,8
CHEMOSAT achieves 10-fold higher peak perfusate drug concentration of melphalan to the liver than systemic administration while controlling systemic exposure and associated side effects.1
CHEMOSAT offers hope for patients suffering from metastatic uveal melanoma. Multiple published clinical studies have demonstrated that CHEMOSAT can meaningfully alter the course of disease and improve patients’ chances of survival. It is a real game changer in the treatment of metastatic uveal melanoma.

Delcath CEO,
Gerard Michel
Delcath are an interventional oncology company.
Delcath are the manufacturers of CHEMOSAT and have over 12 years' experience in the European market. We are committed to improving the lives of patients with primary and metastatic liver cancer. Our in-house clinical team continues to focus on areas such as metastatic uveal melanoma (mUM), intrahepatic cholangiocarcinoma (ICC) & colorectal cancer (CRC)
Oncologist
A medical oncologist will usually be responsible for ensuring that CHEMOSAT is the right treatment approach for their patient. They are experienced in the monitoring of toxicities of chemotherapy and are responsible for the complete medical management of the patient, including, but not limited to, pre- and postoperative care. They may also be responsible for monitoring the patient during the immediate post-procedure period.
Intensivist/critical care specialist
A qualified intensivist, or appropriately qualified critical care specialist, is responsible for providing medical management of the patient in the immediate post-procedure period, during which the patient is in the intensive care unit or step down unit.
lnterventional radiologist
A qualified interventional radiologist with the knowledge, skills, experience, and hospital privileges required to perform advanced vascular interventional procedures.
Nurse
A qualified chemotherapy healthcare professional certified by the site to deliver chemotherapy, such as Interventional Radiology Technician or Registered Nurse.
Perfusionist
A qualified perfusionist is required to establish, monitor, and control the extracorporeal pump and veno-venous bypass circuit.
Anaesthesiologist
A qualified anaesthetist (anaesthesiologist) and/or nurse anaesthetist responsible for the management of sedation, analgesia, respiratory and cardiovascular support.
Pharmacist
A qualified pharmacist, on call during the procedure, to reconstitute the chemotherapeutic agent (melphalan hydrochloride), using national and local safety guidelines. The pharmacist should be aware of the rapid preparation time required for the preparation and administration of melphalan for use with CHEMOSAT.
Assess Eligibility 7
*Patients with 50% or greater tumour burden of the liver on imaging should have a laparoscopy and normal liver biopsied for both visual inspection of liver parenchyma and histological examination of the liver to rule out hepatocellular dysfunction at a subclinical level
Clinical Assessment
Assessment by Expert CHEMOSAT Team
Post Treatment Assessment
Week 1 - blood tests
Week 2 - blood tests
Week 3 - blood tests
Week 6-8 - post treatment MRI to assess response
CHEMOSAT: A 3 Step Process
France
| Publication Date | Document and Revision number | Language |
|---|---|---|
| 26-JUL-2023 | 120057 Rev E | French • Download |
| 26-JUL-2023 | 120057 Rev E | English • Download |
| 21-APR-2022 | 120057 Rev D | French • Download |
| 21-APR-2022 | 120057 Rev D | English • Download |
| 08-MAR-2021 | 120057 Rev C | French • Download |
| 08-MAR-2021 | 120057 Rev C | English • Download |
Spain
| Publication Date | Document and Revision number | Language |
|---|---|---|
| 26-JUL-2023 | 120057 Rev E | Spanish • Download |
| 26-JUL-2023 | 120057 Rev E | English • Download |
| 21-APR-2022 | 120057 Rev D | Spanish • Download |
| 21-APR-2022 | 120057 Rev D | English • Download |
| 08-MAR-2021 | 120057 Rev C | Spanish • Download |
| 08-MAR-2021 | 120057 Rev C | English • Download |
References
References
References
References
Cookies Policy
Using our website
Our website (“chemosat.com”) uses cookies to improve your experience and to provide tailored information.
What are cookies?
Cookies are text files stored on your computer so that when you visit a website it can remember who you are. Using cookies allows websites to remember your preferences and behaviour which enables websites to improve your experience.
Cookies can be set by the owner of the website or in some cases by third-party services the website owner allows to present or store other information or provide other functionality such as data analytics.
How are they used on this site?
Cookies are used to improve the functionality of our website.
This website uses session cookies. Session cookies are used to deliver the basic functions of a website i.e. to allow pages to remember technical changes or selections you may make between pages. Session cookies are temporary cookies and are generally erased when you close your browser.
We may also use cookies set by third parties for other functionalities such as data analytics and improved targeted advertising.
Managing & Removing Cookies
Should you change your mind in the future you always have the option of removing cookies from your browser. Please the below links depending on your browser: